
Published on the 20th August 2012 by ANSTO Staff
Since the 1980s, ANSTO has been a world leader in nuclear waste form research and is now extending its waste form activities into developing nuclear materials for the next generation of reactor technologies. This includes developing materials for advanced nuclear waste forms, and inert-matrix targets for actinide burning.
 |
| Scanning electron micrograph of a ceramic oxide waste form taken on ANSTO’s Zeiss Ultra Plus SEM. Photo courtesy of Joel Davis |
Such materials have the potential to address the worldwide growing stockpiles of plutonium and minor actinides, and to provide long-term nuclear waste management solutions. A key scientific focus is to acquire fundamental knowledge on the crystal chemistry of actinides within suitable materials and to provide an understanding of how the materials behave during the extreme conditions of the advanced fuel cycle.
This research covers current strategies to fabricate and characterise these systems as well as future research directions, and demonstrates the viability of the studied material as a waste form and host for actinides.
Nuclear energy with waste minimisation
To prepare for future generation nuclear systems (socalled Gen IV reactors) and a ‘closed fuel cycle’, it is necessary to provide long-term waste management solutions and address the growing stockpile of plutonium and minor actinides (neptunium, americium and curium) contained in spent nuclear fuel.
Most of the longer term radioactive hazard from irradiated nuclear fuel originates from these four chemical elements (the actinides, see Fig. 1), as well as some long-lived fission products such as iodine and technetium [1]. At ANSTO, we are developing materials which can encapsulate the actinides for use in two different and complementary waste management concepts, (1) conditioning and (2) transmutation:
1. Advanced nuclear waste forms (conditioning)
Actinides are incorporated into the lattice of specific crystalline solids which have high radiation resistance and aqueous durability. These ‘waste forms’ can then be sent to a geological repository for long-term storage.
2. Inert matrix and transmutation targets (transmutation)
Partitioning and transmutation of the actinides potentially reduces the burden on a geological repository. The actinides are removed from the waste (partitioned) and fissioned in a reactor (transmutation). For example, plutonium-239 (half-life of 24,000 years) is transmuted to various fission products, the vast majority of which have significantly shorter half-lives. This concept not only utilses resources (plutonium is an essential energy resource) but also provides enhanced resistance to proliferation. Importantly, this approach would potentially reduce average high-level nuclear waste lifetimes from tens of millennia to a few centuries.
Candidate materials to encapsulate the actinides for transmutation will be required to fulfil a range of criteria including radiation tolerance and desirable thermophysical properties. Not only will these materials experience high radiation fields in the reactor, but also extremely high temperatures. Here we report our investigations into the incorporation of actinides within one such promising candidate material which has use in both the conditioning and transmutation concepts.
Radiation tolerance in crystalline solids
Material choice draws on computer models and inhouse experiments. Element choice is closely tied to the on-going separation research program, resulting in a combined approach to actinide separation and waste form or transmutation target design. This has pointed to zirconia-based compounds which are considered suitable for both the transmutation and conditioning strategies.
Ordered derivatives of cubic zirconia are the pyrochlore-structured zirconates, Ln2Zr2O7 (Ln = lanthanides from La to Gd) and members of this family have been found to have remarkable resistance to amorphisation under ion-beam irradiation [2]. The cationic and anionic ordering within the pyrochlore crystal structure is an extremely important variable and has been linked to this significant radiation tolerance [3].
The ordering can also be affected by actinidedoping, with the oxidation state of the actinide primarily determining its location within the structure. Therefore it is important to study the effect of actinide doping on the ordering in the crystal structure and to determine
the actinide location and oxidation state within the pyrochlore matrix.
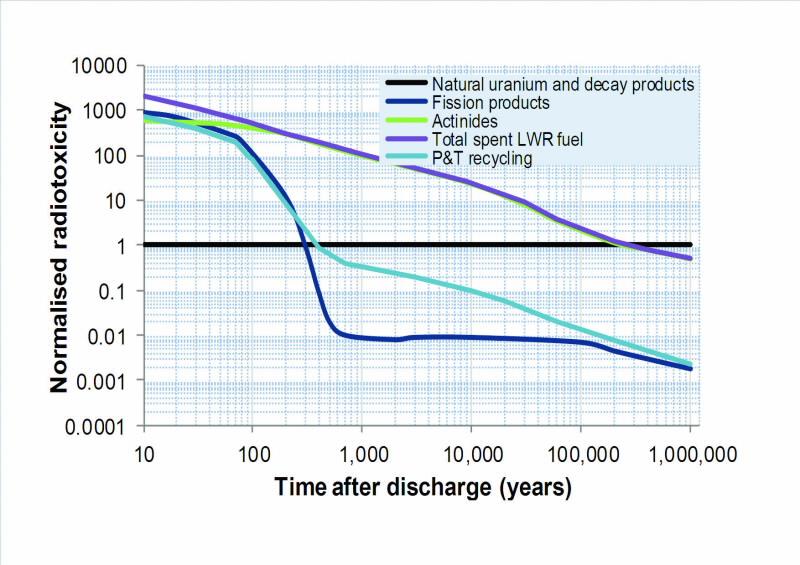 |
| Fig 1. Ingestion radiotoxicity for typical spent UOX fuel (total radotoxicity is broken up into fission products and actinides) and for the same sent fuel following P&T recycling with 99.9% removal of actinides from the waste stream. Values are normalised to natural uranium ore and its decay products. |
Incorporating actinides into the crystal structure
Uranium- and plutonium-incorporated samples are prepared in designated facilities within ANSTO’s Institute of Materials Engineering. Small samples (~1 g) are prepared and are sectioned for various characterisation techniques. X-ray diffractometry and scanning electron microscopy are used to determine the phase composition, sample purity and microstructure.
The porous nature of the samples is clearly visible in the scanning electron microscope images (Fig. 2(a) an (b)), however high density is an essential requirement for application as transmutation targets and durable waste forms. This has formed the catalyst for the group to develop a novel, simple, low-temperature chemical synthetic route for the preparation of dense, homogeneous zirconate ceramics.
The method allowsintimate mixing in the aqueous phase at ambient temperature and produces zirconate pyrochlores with high density. Fig. 2(c) illustrates our extremely successful processing result.
The actinide oxidation state in each sample can be determined using a combination of diffuse reflectance and X-ray absorption near edge structure (XANES) spectroscopies. See Fig. 3 for typical XANES spectra (carried out on the Australian Synchrotron) for two such uranium-doped matrices sintered in air. We found that all the air-sintered samples were consistent with a U6+ oxidation state, whilst those sintered in argon or H2/N2 atmospheres revealed mixed U5+/U6+ oxidation states. Interestingly, this occurred even when Ca2+ was added to try to charge balance for U4+.
There are two cationic crystallographic sites in the pyrochlore structure and the actinide element can potentially be accommodated on either. Clues provided from oxidation-state information along with refinement of high-resolution X-ray diffraction data can be used to determine the location of the actinide within the matrix structure, i.e. on which cationic site does the actinide sit? Transmission electron microscopy can then provide specific information on the cationic/anionic ordering in the structure following actinide doping, as shown in Fig. 4.
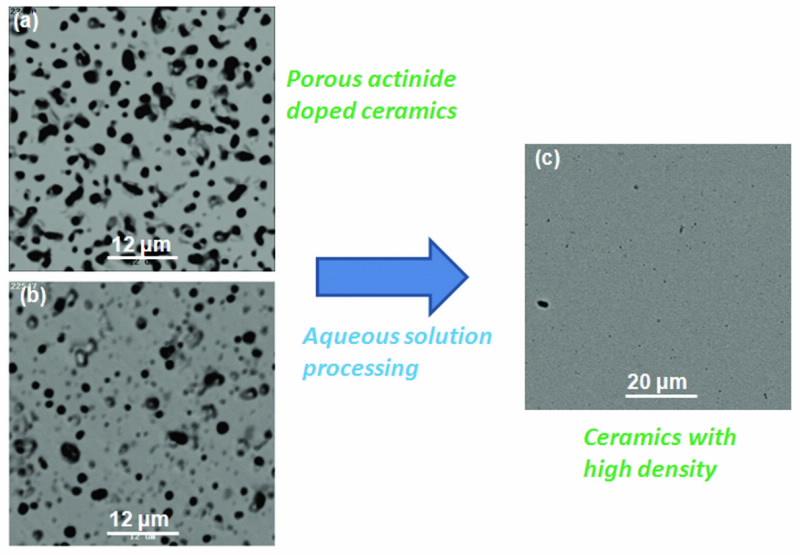 |
| Fig 2. Backscattered scanning electron micrographs (a) uranium-doped and (b) plutonium- doped ceramic matrices after sitering at 1400oC. The dark areas are pores. Micrograph (c) shows the increased density achieved for a sample formed using our novel chemical synthesis approach after sintering at a similiar temperature. |
Where to from here?
Considering the ability of the pyrochlore-structure phase to accommodate uranium and plutonium, zirconate pyrochlores are promising host matrices for use as actinide transmutation targets and specialised nuclear waste forms. In future work we plan to investigate the radiation tolerance of this system through the synthesis and characterisation of 238Pu-doped samples.
The long-term effects of α-decay events can be simulated by incorporating short-lived actinides, such as 238Pu (half-life of 87.7 years) [4]. This provides information on the radiation tolerance to α-decay events at accelerated
dose rates in laboratory time scales (~2 years) and will place ANSTO amongst a handful of organisations worldwide that can undertake this type of research.
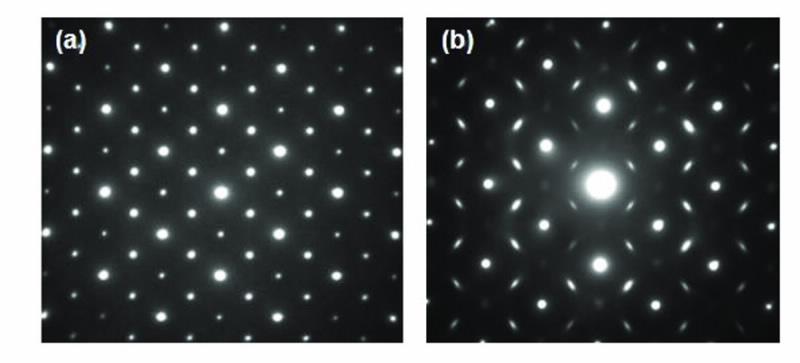 |
| Fig 4. Selected area diffraction patterns of two actinide-doped zirconate pyrochlores, viewing down the [110] zone axis. (a) shows sharp spots characteristic of the ordered pyrochlore, while (b) shows diffuse scattering indicating incommensurate ordering within the structure. |
References
- “Potential Benefits and Impacts of Advanced Nuclear Fuel Cycles with Actinide Partitioning and Transmutation”, 2011 Nuclear Energy Agency report available online at: http://www.oecd-nea.org/science/reports/2011/6894-benefits-impactsadvanced-fuel.pdf.
- Ewing, R. C.; Weber, W. J.; Lian, J. J. Appl. Phys. 2004, 95, 5949.
- Zhang, J.; Lian, J.; Zhang, F.; Wang, J.; Fuentes, A. F.; Ewing, R. C. J. Phys. Chem. C 2010, 114, 11810.
- Weber, W. J.; Ewing, R. C.; Catlow, C. R. A.; Diaz de La Rubia, T.; Hobbs, L. W.; Kinoshita, C.; Matzke, Hj.; Motta, A. T.; Nastasi, M.; Salje, E. H. K.; Vance, E. R.; Zinkle, S. J. J. Mater. Res. 1998, 13, 1434.