Building on comprehensive work of a former ANSTO researcher Prof Suzanne Smith, Clarity Pharmaceuticals, a radiopharmaceutical company focused on the treatment of serious disease, has progressed its lead product SARTATE™ from the lab into the clinic for treatment of a range of cancers including neuroendocrine tumours and childhood cancers. The product, a promising molecular imaging agent for cancer diagnosis and treatment has been developed through close collaboration between Australian academia, ANSTO and business.
In a prescient statement in her 2004 paper, Prof Smith speculated on the potential role of a new “chelator” technology as a powerful molecular imaging agent to diagnose disease, evaluate response to treatment and, ultimately, be used as a therapeutic. The vision of Prof Smith has in effect come to fruition with the development of SARTATE™.
There have been many contributors to the development of SARTATE™ from collaborating institutions over the years since Smith’s seminal research including Clarity Pharmaceuticals, who own SARTATE™, the School of Chemistry and Bio21 Institute of Molecular Science at the University of Melbourne, and the Peter MacCallum Cancer Centre. ANSTO is a shareholder of Clarity Pharmaceuticals.
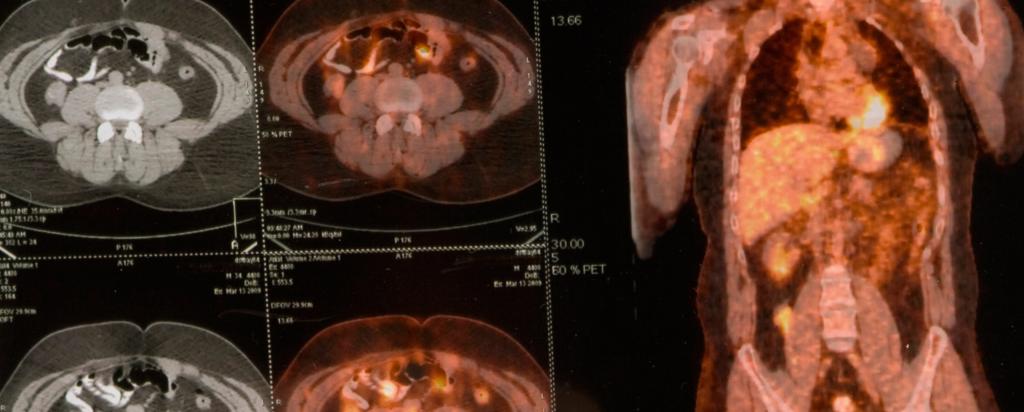
In a recent statement, Clarity Pharmaceuticals announced that SARTATE™ has completed a proof of concept clinical trial for neuroendocrine tumours and the results would be presented by collaborators from the Peter MacCallum Cancer Centre at the Society on Nuclear Medicine and Molecular Imaging meeting in the USA in June 2016.
The origins of SARTATE™ go back to pioneering work by Prof Smith, Prof Alan Sargeson and colleagues from the Australian National University in assessing the most suitable chelator that could be used with isotopes of copper. A chelator is essentially a cage that holds the copper tightly inside it. These chelators were interestingly named “sarcophagines”, in reference to an Egyptian sarcophagus.
Copper isotopes became of interest for use in molecular imaging at the time because they possessed a number of desirable factors. They have optimal half-lives and energies suitable for the burgeoning class of drugs called biologics, particularly monoclonal antibodies and peptides.
These biologics were being developed at the time for use as therapies for cancer and other serious diseases. The sarcophagine chelators provided the chemistry that allows them to link to these antibodies or peptides.
Prof Smith suggested that the sarcophagine technology could be a convenient way to image and check if these biologics were actually going to disease sites within the body.
Prof Smith and colleagues from the Australian National University went on to evaluate a number of antibodies with the sarcophagines, which included fragmented antibodies for uptake into neuroblastoma tumours in murine models, published in Proceedings of the National Academy of Science Journal in 2007. In her work, she assessed a range of potential chelators with copper isotopes using a range of biochemical, molecular and imaging techniques.
The problem identified in Prof Smith’s research was that existing chelators leak copper and thus leave patients vulnerable to side effects. In an effort to find a better solution, Prof Smith commenced research and development of SarAr, a novel cage that has the ability to completely encapsulate copper isotopes and demonstrate in vivo thermodynamic stability, kinetic inertness and rapid clearance from the body.
Due to the synthesis of sarcophagine cages being considerably simpler than other ligands, it made them readily adaptable to large scale production.
Since the antibodies or peptides could be seen to accumulate on disease sites in the body, they could be measured. The data could be used to determine the response to treatment and therapeutic strategies and personalisation of dose.
This is fundamentally attractive in paediatric nuclear medicine where dose calculations are difficult yet very important. Clarity recognises paediatrics as a key area to investigate and conduct trials.
Clarity’s Managing Director and Founder Dr Matt Harris says, “We believe in personalised medicine and the development of new therapies that are more effective and have less side effects. At Clarity, we recognised that radiopharmaceuticals and the sarcophagine technology from ANSTO had this potential.”
By 2008, SarAr had evolved into a mature technology and its potential for Cu-64 PET imaging far exceeded the expectations of its proponents.
Smith and collaborators also anticipated that SarAr’s ability to attach to molecules or nanoparticles could be used to conduct risk assessments of drugs in clinical trials.
A great deal of work on the SarAr technology has been done in the years since Smith’s fundamental research by Associate Professor Paul Donnelly of the School of Chemistry and Bio21 Institute of Molecular Science and Biotechnology, University of Melbourne and Clarity Pharmaceuticals to develop the product SARTATE™. Prof Rod Hicks, a world leader in the field, at the Peter MacCallum Cancer Centre supervised the proof-of-concept clinical trial of SARTATE™ as a novel cancer diagnostic for neuroendocrine tumours.
Clarity has commenced further research on SARTATE™ as a cancer therapy for neuroendocrine tumours and neuroblastoma in children.
Dr Alan Taylor, Clarity’s Chairman commented: “SARTATE’s successful development to date is a great example of how collaboration between academia and business improves Australian innovation. By building on Suzanne’s research and working together with the brightest scientists and clinicians in the field, we are hoping to translate this work into internationally successful treatments that will ultimately help save lives and reduce treatment side effects”.