
Published on the 21st February 2012 by ANSTO Staff
The translocator protein is a vital component in stress and anxiety regulation. As cannabis has the potential to alter anxiety, stress levels and also trigger psychosis and schizophrenia, this study investigates the influence of continuous administration of cannabinoid on the translocator protein in both adult and adolescent rats.
Since the translocator protein is mainly expressed outside of the brain, a variety of organs were studied. The results show that cannabinoids have a negative impact on the testicular translocator protein.
However importantly, this finding was only observed in the adult rats and not in the adolescent rats. The implications of observing a specifically different effect in the adult compared to adolescent rats suggest adults were most affected by cannaboid treatment whereas adolescent rats showed little change.
It is possible that the failure to regulate the translocator protein in adolescents may lead to diseases such as schizophrenia and psychosis in later life.
 |
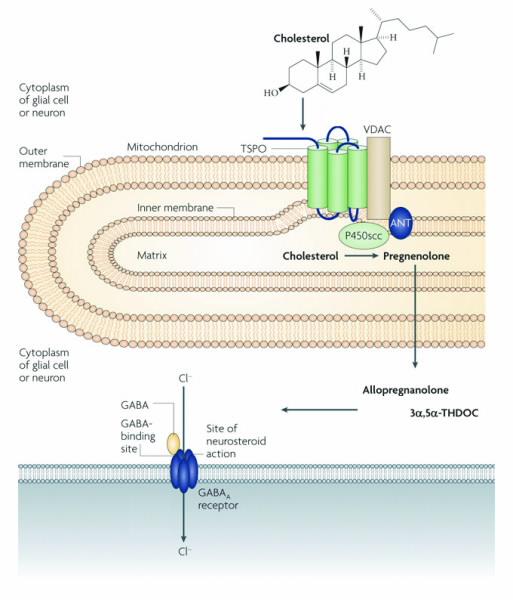
| Fig 1. A schematic diagram of how TSPO might modulate stress and anxiety. Within the brain, cholesterol is transported into the matrix side of the mitochodrion with help from TSPO and other proteins. Once inside, side-chain-cleaving cytochrome P450 enzyme (P450scc) converts cholesterol into pregnenolone. Here it diffuses into the cytoplasm and is converted by a series of other enzymes (not shown) into allotetrahydrodeoxycorticosterone. These neurosteroids are the able to modulate the binding of GABA to GABAA recptors which in turn alters the receptors function nd affect stress and anxiety responses. Figure appropriated from [3]. |
The translocator protein
The translocator protein (TSPO) is an ancient protein, with analogues found in almost all living organisms studied to date. In mammals, it is expressed in nearly all tissues with important physiological and pathophysiological roles.
Currently, known roles and functions of this protein include cholesterol transport, steroid production (steroids are organic compounds found in plants, animals and humans), stress regulation, cell proliferation, apoptosis (normal process leading to cell death), heme (a large ring-shaped molecule with an iron atom at its centre) transport and immune modulation [1].
However, perhaps the best studied function is TSPO’s role in steroid production and stress regulation. It has been recognised that TSPO is involved in the transport of cholesterol across the mitochondrial membranes (mitochondria are the energy productive sites of a cell).
Once transported inside the matrix side of the mitochondria, enzymes begin converting cholesterol into steroid precursors. Thus, the ability of the TSPO to assist in the transport of cholesterol is critical for the health and development of an organism; this is particularly so as the cholesterol transport process is also the rate limiting step of all new steroid production [2].
Studies on the impact of stress and anxiety have noted that TSPO expression is dramatically decreased upon exposure to prolonged periods of stress.
The type of stress does not appear to be limited to psychological stress, but also applies to physical stress including forced swimming and electric shocks [1]. In this context, TSPO appears to be a general marker for prolonged stress, with decreases in expression correlating to the degree of stress experienced. The mechanistic process for which TSPO contributes towards stress and anxiety regulation is still being investigated.
One theory is that since TSPO is involved in the rate-limiting step of cholesterol transport, the expression of TSPO is able to regulate steroid production within the brain.
These neurosteroids, such as allopregnanolone and allotetrahydrodeoxycorticosterone, have been shown to regulate the primary inhibitory neurotransmitter, GABA transmissions, within the brain.
The GABA neurotransmitter system is well known for its stress and anxiety regulating properties (Fig. 1) [3]. Other researchers have suggested the influences of peripheral organs including the kidneys and adrenal glands, citing the high expression of TSPO in the periphery compared to the brain.
Studying chronic cannabinoid treatment
Understanding the roles of TSPO particularly in steroid production, we sought to investigate the effect of a chronic cannabinoid treatment on the expression of TSPO. In this light, cannabinoids are interesting not only due to their psychoactive and anxiety altering nature, but also due to their peripheral effects, namely those on testosterone production and sperm production.
Cannabinoids have negative effects on both testosterone production and sperm production. Previous literature reports reduced testosterone production after both acute and chronic exposure to either cannabis and/ or cannabinoids. Further, sperm production is decreased along with increases in the incidences of abnormalities and defects.
We used a rat model [4] previously established at ANSTO, to examine the effects of chronic cannabinoids on the expression of TSPO in a variety of organs including the spleen, kidneys, liver and testes. Further, we also examined the effect on adult and adolescent rats since cannabinoids are known to affect adults and adolescents differently.
Different effects for adults and adolescents
Administration of chronic cannabis (daily over two weeks) clearly elicited a general stressful response, with dramatic decreases in weight across both adult and adolescent treated groups (Fig. 2). Our investigations into the expression of TSPO confirmed that changes in expression were indeed stress-stimulus dependent.
We failed to find any changes in the renal, liver or spleen tissues; results from the renal tissues are particularly interesting as they are the classical responders to either electric shock or forced swim stress. In response to cannabinoid treatment, variations within the testicular tissues were found.
However this was not too surprising since it has already been confirmed that cannabinoids produce alterations within testosterone levels [5]. It does confirm that cannabinoids act on a very basic level at suppressing testosterone production through the TSPO.
However, what is much more interesting and surprising is that we found a differential effect between adult and adolescent rats (Fig. 3).
In most cases, early-age drug use, particularly during adolescence, can have long lasting negative impacts resulting in neurological and behavioural deficits. Here, we found that adults were most affected by cannabinoid treatment whereas adolescent rats showed little difference from controls.
It is possible that, with respect to the effect of cannabinoids on TSPO expression, it is the failure of regulation that may lead to these deficits later in life. Given that cannabis consumption is strongly linked with the development of schizophrenia and psychosis [6], the inability of adolescent animals to regulate testosterone levels through the TSPO may represent a novel avenue of future research in elucidating the developmental causes of various psychiatric illnesses.
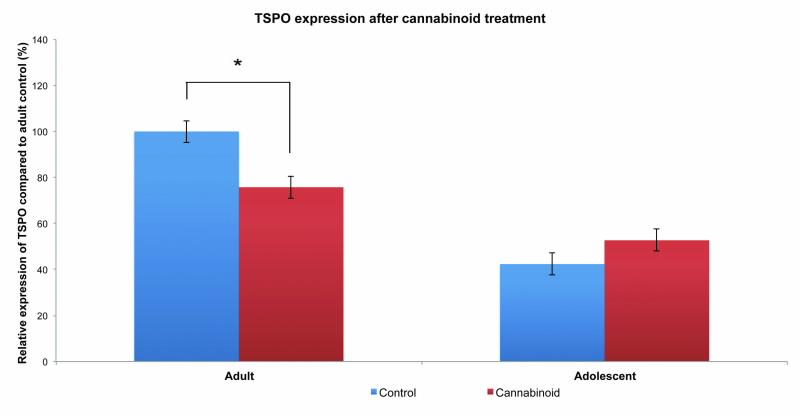 |
| Fig 3. Relative expression of TSPO in testicular tissues following chronic cannabinoid treatment. Expression is significantly different in the adult group with decreases in cannabinoid treated animals (*p<0.01). In contrast, no significant differences are seen in the adolscent group. Expression is relative to adult control. |
Authors
Ronald Chan1,2, Winnie Kam1, Guo Jun Liu1,2, Katerina Zavitsanou1,3, Richard Banati1,2
1ANSTO, 2University of Sydney, 3Schizophrenia Research Institute, Sydney
References
- Gavish. M., Bachman. I., Shoukrun. R., Katz. Y., Veenman. L., Weisinger. G., Weizman. A., Enigma of the peripheral benzodiazepine receptor, Pharmacological Reviews, 51(4), 629-650 (1999).
- Pandak. W.M., Ren. S., Marques. D., Hall. E., Redford. K., Mallonee. D., Bohdan. P., Heuman. D., Gil. G., Hylemon. P., Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes, Journal of Biological Chemistry, 277(50), 48158-48164 (2002).
- Rupprecht. R., Papadopoulos. V., Rammes. G., Baghai. T.C., Fan. J., Akula. N., Groyer. G., Adams. D., Schumacher. M., Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders, Nature reviews. Drug Discovery, 9(12), 971-988 (2010).
- Dalton. V.S., Wang. H.Q., Zavitsanou. K., HU210-induced downregulation in cannabinoid CB1 receptor binding strongly correlates with body weight loss in the adult rat. Neurochemical Research, 34(7), 1343-1353 (2009).
- Gorzalka, B.B., Hill, M.N., Chang, S.C., Male–female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function, Hormones and behavior, 58(1), 91-99 (2010).
- Smit. F., Bolier. L., Cuijpers. P., Cannabis use and the risk of later schizophrenia: a review, Addiction, 99(4), 425-430 (2004).