Studying enzyme mechanisms using deuterated substrates
Published by NDF staff 13th October, 2022
The NDF is providing deuterated substrates to enable researchers to study enzyme binding. This is important for designing enzymes for applications as drug targets and as biocatalysts.
These studies demonstrate the broadening user base for the National Deuteration Facility, with requests to synthesise deuterated molecules for neutron as well as non-neutron based applications.
- Enzymes offer great potential for a variety of biotechnological applications; for example as biocatalysts for performing oxidations in the synthesis of natural products, or as targets for the development of therapeutics.
- Studying the structure and mechanism of action of enzymes is important for progress to be made in these areas.
- The National Deuteration Facility has synthesised and provided deuterated molecules to Associate Professor Stephen Bell at the University of Adelaide for investigations using cytochrome P450 enzymes.
- These studies demonstrate the increasing demand for the National Deuteration Facility to synthesise deuterated molecules for non-neutron based applications; in this instance for mass spectrometry and the kinetic isotope effect.
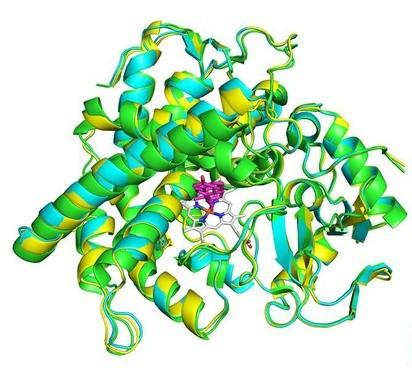
- In one study, deuterated cholesterol was used to investigate the steroid binding in CYP142 cytochrome P450 enzymes. These enzymes are proposed to be involved in steroid metabolism in mycobacteria (the type of bacteria responsible for tuberculosis and leprosy). Identifying molecules that can target these enzymes is important for drug development and overcoming drug resistance. It was demonstrated using GC-MS analysis of the competitive oxidation of a mixture of protiated and deuterated cholesterol by the enzyme, MmarCYP142A3, that there is no preference for the protiated cholesterol. This demonstrated that C−H bond abstraction is not a significant rate determining step in the oxidation reaction.

The overlay of X-ray crystal structures of the CYP142 enzymes.
The choleste-4-en-3-one substrate is shown in magenta.1
- In another study, the group of Associate Professor Bell aimed to explore the oxidation mechanism mediated by an engineered P450 peroxygenase variant. A deuterated substrate (4-(methoxy-d3)benzoic-d4 acid) was prepared by the NDF to probe the kinetic isotope effect. The absence of a kinetic isotope effect has provided mechanistic insight into this transformation.
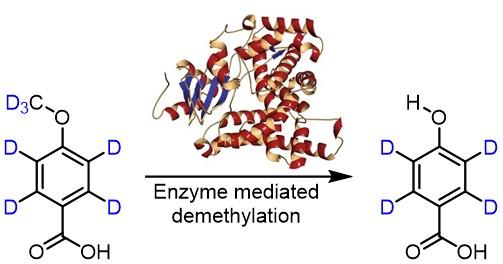
The enzyme mediated demethylation reaction of 4-(methoxy-d3)benzoic-d4 acid.
NDF staff contributing to this work: Dr Rob Russell, Dr Michael Moir
References
The Structures of the Steroid Binding CYP142 Cytochrome P450 Enzymes from Mycobacterium ulceransand Mycobacterium marinum
Ghith, Russell, Bell et al. ACS Infect. Dis. 2022, 8, 8, 1606–1617.Selective Oxidations Using a Cytochrome P450 Enzyme Variant Driven with Surrogate Oxygen Donors and Light
Lee, Podgorski, Moir, Gee, Bell. Eur. J. Chem. 2022
Enquiries

Dr Tamim Darwish, NDF Leader
The National Deuteration Facility is partly supported by the National Collaborative Research Infrastructure Strategy – an initiative of the Australian Government.
