Particle Induced Gamma Ray Emission
Basic Physical Principles
When a charged particle (typically protons) approaches the nucleus of a target atom, the coulomb force usually repels it. However, when the incident particle has enough energy to overcome the repulsive coulomb force, a charged particle then penetrates through the electrostatic barrier into the nucleus, resulting in interactions with the nuclear forces.
During that process, a number of interactions occur, depending on the energy of the incident particle and the type of target nucleus. Typically, a nuclear reaction will occur, resulting in the emission of high energy x-rays (x-rays emitted from the nucleus are for historical reasons called gamma rays) and other nuclear particles.
In the case of the PIGE technique, emitted gamma rays are of particular interest as their energies are characteristic of the element and are therefore used to fingerprint elemental composition while yields are used to quantify elemental concentrations.
Detection
The detection of the emitted gamma rays is usually done by large volume Ge detectors. PIGE is typically run in conjunction with PIXE and RBS and is used to quantify concentrations of low Z elements such as: Li, F, Na, Mg and Al.
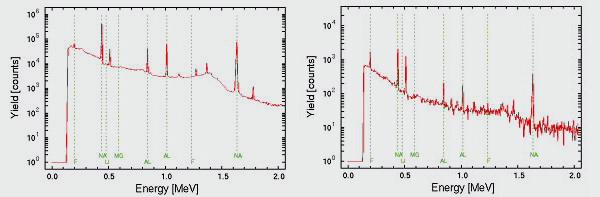
Detection limits vary from element to element but it is typically between 10 and 100 ppm. A typical gamma spectra of an archaeological sample and an aerosol sample are shown below.

Figure 1 (left) PIGE spectrum of an archaeological sample (obsidian glass)
Figure 2 (right) PIGE spectrum of an aerosol sample
Capabilities
The requirement for a weak coulomb force thus imposed restrictions on the charge of the incident particle and of the target atoms - they both have to have low nuclear charge. This restricts the PIGE method to the measurement of light elements in the periodic table (e.g. lithium, fluorine, sodium, magnesium and aluminium).