
Published on the 1st December 2025 by ANSTO Staff
Key Points
-
International researchers have found a better way to separate two rare and important gases, xenon and krypton, using a zeolite material
-
Industry will benefit as the gases are often combined in chemical, petrochemical, metallurgical, and environmental processes
-
Data from the Powder diffraction beamline at the Australian Synchrotron provided a detailed picture of the material’s atomic structure
An international team of researchers have found a better way to separate two rare and important gases—xenon and krypton—that are often combined in chemical, petrochemical, metallurgical, and environmental processes.
The separation of these two gases is very challenging because of their very similar sizes and physical properties.
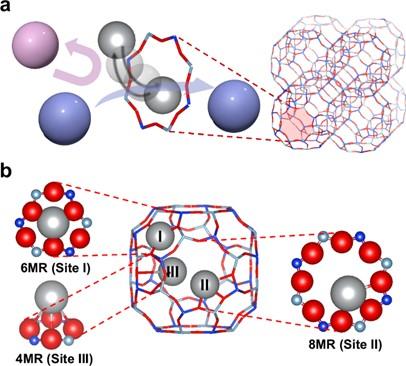
Instead of using the usual energy-heavy freezing method (cryogenic distillation), they developed a material with special properties, zeolite, which works like a sponge with tiny holes.
Ions of silver move around inside the zeolite; a material commonly used in industry. These ions strongly interact with xenon and exclusively get xenon trapped while krypton remains for the most part outside the material.
By adjusting the number of silver and calcium ions, they made the process faster and more efficient.
The xenon is captured much more easily than krypton—in fact, over 1,600 times more likely. Additionally, the material can be reused without losing strength.
This method could replace expensive cooling systems with a cheaper, energy-saving solution for industry.
The team used the Powder diffraction beamline at the Australian Synchrotron to get a detailed picture of the material’s atomic structure at different temperatures.
The data was combined with computations methods to create 3D models to depict where the atoms and ions sit inside the material.
“This understanding of how the material works at the atomic level, is key for improving its performance” said Dr Qinfen Gu, Principal Beamline Scientist, a co-author on the paper that was published in Nature Communications.
The research was led by scientists from the City University of Hong Kong, Zhejiang Normal University (China) and Fujian Normal University (China) with collaborators from Tsinghua University (China), and Tongji University (China).

