Published on the 24th November 2023 by ANSTO Staff
In a rare collaboration, two scientists, who are brothers working in unrelated disciplines, combined complementary expertise to tackle a chemical problem relating to the use of silicon in electronic devices.

Leader of the National Deuteration Facility, Dr Tamim Darwish (above right), suggested to his brother, Dr Nadim Darwish (above left), a Senior Lecturer with expertise in molecular electronics at Curtin University, that deuterating silicon might improve its properties.
Dr Tamim Darwish is very familiar with the unique properties of deuterium, an isotope of hydrogen that is used to replace hydrogen in molecules and the focus of work at the National Deuteration Facility (NDF).
This facility at ANSTO is a world leader in deuteration for research applications, and they specialise in providing bespoke deuterated molecules and labelling techniques.
The results of research published in Applied Materials and Interfaces reported enhancements to the properties of silicon when hydrogen was replaced with deuterium on the surface layer.
In recent years, there's been significant interest in a technology that combines silicon and organic molecules for various applications such as sensors, solar cells, power generation, and molecular electronic devices.
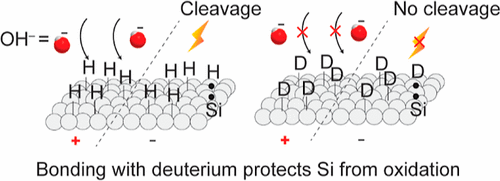
The challenge with this technology has been that the surfaces made of silicon and hydrogen (Si−H surfaces), which are crucial for building these devices, are prone to oxidation. This oxidation can harm the stability of the devices both mechanically and electronically.
In this study, the Darwish brothers and co-workers found that if hydrogen is replaced with deuterium, creating Si−D surfaces, these surfaces become much more resistant to oxidation when exposed to either positive or negative voltages. Si−D surfaces demonstrated more stability against oxidation, and their electrical characteristics were more consistent compared to Si−H surfaces.
The investigators recommended using Si−D surfaces instead of Si−H surfaces in applications that require non-oxidized silicon surfaces, such as electrochemical biosensors, silicon-based molecular electronic devices, and silicon-based triboelectric generators.
The significant surface isotope effects reported in this study have implications for the design of silicon-based devices, molecular electronics, and power generation devices based on silicon.
Additionally, these findings impact the interpretation of charge transport characteristics in such devices.