
Published on the 20th October 2015 by ANSTO Staff
The creation of the first healthy mouse without the evolutionary conserved mitochondrial translocator protein, long thought to be essential for life, is an important scientific breakthrough. Developed at ANSTO, it paves the way to new diagnostics and treatments for inflammation, dementia, obesity and cancer.
 |
| The content of this article appears in a Nature special publication: Spotlight on Australia ANSTO seeking partners to accelerate the discovery |
To aid the systematic study of the translocator protein (TSPO) in membranes, our scientists and colleagues at the Brain and Mind Centre at the University of Sydney have been using Australia’s landmark and national nuclear science infrastructure, which includes ANSTO’s neutron reflectometer instrument (Platypus), to develop diagnostic tools, such as radioligands for positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging.
This is part of a strategic interdisciplinary research project aimed at understanding the function of this abundant and highly regulated, ancient mitochondrial protein.
The broad diagnostic and therapeutic importance of the TSPO stems from the observation that its cellular expression increases dramatically during inflammation, dementia, obesity and cancer, and also appears to play a role in behavioural conditions, such as anxiety.
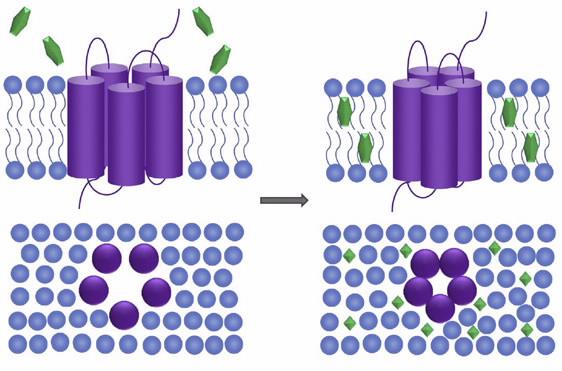
Moreover, the interaction of small molecule drugs with lipid membranes can modulate the conformation and function of resident membrane proteins such as TSPO (see image above). Thus, in addition to its role as a diagnostic biomarker of disease progression, TSPO is also attracting attention as an important therapeutic target.
As of 2014, there have been well over 30 clinical trials involving diagnostic and therapeutic aspects of the TSPO in disease conditions ranging from inflammation to neurodegeneration and behavioural illnesses
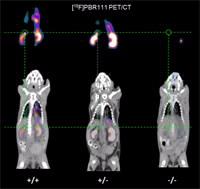
In their latest study (Nature Communications 5, 5452; 2014), the team, led by Richard Banati, reported the existence of healthy global TSPO gene knock-out mice and confirms the presence and absence of the gene by in vivoimaging (see image right).
The team’s findings came as a surprise since previous observations had suggested that the loss of the TSPO, in line with its pivotal role in steroid synthesis, was embryonal lethal.
Consequently, the new findings have generated a lively debate about some of the most basic aspects of physiology, such as the synthesis pathways of steroids, for which the TSPO has long been thought to be one of the rate-limiting steps.
As part of their ongoing investigations, ANSTO scientists observed alterations in the energy metabolism in microglia cells, the brain’s resident immune cells.
With a nod to the birthplace of the mice and the fact that mitochondrial energy production may be altered, the scientists named their mouse Guwiyang Wurra, which means ‘fire mouse’ in the local indigenous Dharwal language.
“Obviously, understanding the true role of the TSPO is of fundamental scientific interest and a global gene knock-out model is important for systematic loss-of-function and mechanisms of compensation studies,” says Banati.
“However, while we are trying to develop such a model, we have already demonstrated the immense practical utility of this animal model for TSPO drug development, particularly for assessing drug selectivity in life and off-target effects that may cause toxic or other effects.”
The researchers are especially excited about the possibility of creating tailored cancer models. For example, the TSPO knock-outs could be used to study syngeneic brain tumours that have retained their wild-type TSPO, and observe the tumour response to treatment in virtual isolation from the surrounding tissue under realistic in vivo conditions.
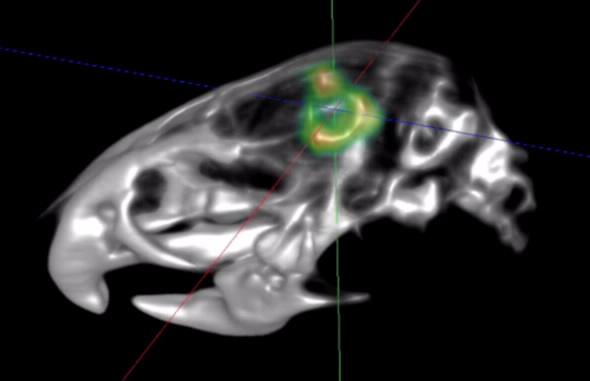
The team were able to generate high resolution and contrast micro PET and computed topography images (using TSPO ligand PBR111) to show TSPO expressing tumour cells in a TSPO knock-out animal (see image above) and in a human brain.
“Such biologically background-free imaging in the living animal has never been done before,” comments Banati.
ANSTO recognises that effective partnering can accelerate realising the potential of discoveries, turning them into innovative products more quickly and cost effectively than is possible through purely in-house development.
ANSTO is therefore actively seeking collaborative partners to use the TSPO knock-out mouse model (C57BL/6-Tspotm1GuWu) to determine the action of new compounds, including the repurposing of existing compounds and the development of novel therapeutic approaches.
ANSTO seeking partners
Partnership opportunities include:
• TSPO KO mouse: strong intellectual property portfolio to develop collaborations
• Ligand screening: TSPO KO and wildtype mice to evaluate TSPO ligands (ligand does or does not bind to the TSPO)
• Drug development: validate the biological pathways involving theTSPO; identify lead compounds that have therapeutic effects
• Preclinical: lead candidates developed using the TSPO KO mouse are established or are being developed in a preclinical environment
The work on the knock-out mouse model and the extensive medicinal chemistry research at ANSTO is part of a broader portfolio that ranges from structural biology using synchrotron X-ray, neutron scattering and cancer therapeutics, to environmental health studies using the largest particle accelerator infrastructure in the Southern Hemisphere.