An approach that combines advanced neutron and X-ray scattering techniques and theoretical modelling has provided insights into the dynamic interactions in promising materials for sodium-ion batteries.
Although much progress has been made using scientific techniques to capture atomic level information of battery materials and their evolution while operating, a study published in the Journal of the American Chemical Society reports on the dynamic interactions of atoms in a battery material.
“Seeing how things move and how motion occurs is actually quite an important parameter for materials used in batteries,” said A/Prof Neeraj Sharma, co-author of paper and an expert on batteries.
“In an operating battery that is charging and discharging, atoms will be moving all over the place.
“The established approach is to use something called impedance to look at movement as a whole or at the bulk-scale, and then deconvolute to estimate the contribution of the individual components.
“What we have shown here is that it is possible to focus in on different atoms, in this case, the sodium ions that diffuse through the structure. We found that these ions were coupled to phosphate anions that rotate.
“It was not only the sodium ions that were moving but the phosphate framework as well in a coordinated paddle wheel-type motion,” said A/Prof Sharma.

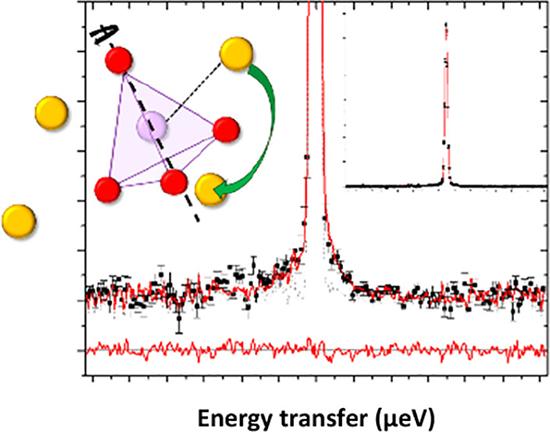
QENS spectra and visual representation of collective motion Credit: J. Am. Chem. Soc. 2021, 143, 41, 17079–17089
“When you have a clear picture of this motion, you can start to think about the overall dynamics of the system.”
Quasi-elastic neutron scattering (QENS) experiments on the material, magnesium-doped sodium orthophosphate (Na3–2xMgxPO4 ), using the high-resolution backscattering spectrometer Emu at ANSTO's Australian Centre for Neutron Scattering provided insights into the sodium ion diffusion mechanism and confirmed the dynamic orientational disorder of the phosphate atoms.
First author, Dr Emily Cheung, a PhD candidate at the time from the University of NSW, carried out the experiments using Emu under the supervision of senior instrument scientist Dr Nicolas de Souza.
Preliminary investigations to characterise the structure of the material were undertaken by co-author Prof Max Avdeev of ANSTO and the University of Sydney using neutron powder diffraction on the high-resolution powder diffractometer Echidna before the experiments on Emu.
“We usually apply QENS to study the dynamics of hydrogen atoms and organic molecules in diffusion processes. The signal from sodium atoms is more challenging but resolvable,” said Dr de Souza.
“When you have a material that is a good conductor, you don’t necessarily know how many mobile ions there are,” said Dr de Souza.
“Being able to determine that and determine whether the diffusion was fast or slow with empirical data from QENS and theoretical calculations is very useful,” he added.
The diffraction data suggested six possible orientations of the phosphorous atoms that were bound to oxygen and sodium atoms.
The investigators estimated that 2 out of 12 sodium cations per unit cell participated in long-range diffusion.
The research also proposed two possible diffusion pathways, that were dependent on the coordination of the phosphate-sodium binding.
One of these pathways was found to be the preferred diffusion model using a theoretical framework that was consistent with the experimental data.
“It was necessary to distinguish any phosphate-bound sodium from other sodium that does not participate in phosphate rotations,” said A/Prof Sharma.
To do this, the investigators developed a theoretical model of long-range sodium ion diffusion using a jump matrix construction for a simplified system.
The approach was first conceived in the 1970s and 1980s but became less used because of the mathematical complexity of new materials designed for next-generation batteries.
Both A/Prof Sharma and Dr de Souza affirm that the modelling developed to identify the contributions of sodium atoms, that scatter neutrons both coherently and incoherently, was a considerable achievement by the first author Dr Emily Cheung.
“We have made some progress in identifying why the material is a good conductor,” said Dr de Souza.
“The theoretical framework that has been developed can be used by investigators in studies on other materials,” he added.
ANSTO Instrument scientist Dr Anton Stampfl is also a co-author on the publication.
Scientists from the University of California San Diego also contributed to the research providing valuable insight into the materials, their synthesis and their performance parameters.
DOI: https://doi.org/10.1021/jacs.1c06905









