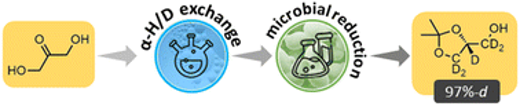

Studies in selective microbial deuteration for chiral molecules
Published by NDF staff, 20th June, 2025
Combining synthetic organic chemistry and microbes to perform enzymatic transformations allowed the enantioselective synthesis of important chiral molecules.
An NDF-led study investigated development of potential cost-effective methods for reductive microbial deuteration (utilising yeast strains) to produce crucial chiral building blocks for medicinal and analytical applications with a high degree of isotopic labelling and enantioselectivity.

A two-part strategy employing both synthetic chemistry and deuterative microbial reduction gave >95% backbone deuteration
Enantiomers are compounds with chemical structures that are non-superimposable mirror images. While their structures appear similar, enantiomers often have very different biological properties. Selective deuterium incorporation can beneficially alter the metabolic, chemical and physical properties of molecules with new techniques for organic molecule selective deuteration essential for meeting growing demand for deuterated compounds in fields such as pharmaceuticals, neutron science and deuterated standards for analytical chemistry. Pharmaceuticals and studies of biological or biomimetic systems utilising neutron techniques along with application of deuterated standards for quantitative analysis of biomolecules (e.g. lipidomics), generally require a particular enantiomer of a chiral compound. Enantioselective deuteration is a challenge with the synthesis of enantiomerically pure compounds often not trivial by synthetic chemistry means and such compounds can be difficult to source commercially.
Microbial transformations are generally enzyme-catalysed and so offer a way to perform deuterative enantioselective reactions, with many synthetic organic chemists familiar with using Baker's yeast for chemical transformations. Such microorganisms can generate required catalysts and redox cofactors from deuterated growth medium and unlike non-biological systems, one enzyme enantiomer is generally available, leading to one enantiomer of the reduced product.
In this study, selected yeast strains were grown in D2O and fed on cheaper deuterated carbon sources and then exposed to precursors of interest. Deuterative microbial reduction was used in one step of the successful synthesis of one of two structures targeted in the study, the versatile building block solketal (2), which has a role in the synthesis of medicinally-relevant compounds and is used in the synthesis of all triglycerides and glycerophospholipids.
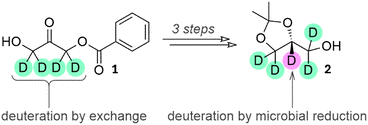
The teal deuterons were incorporated using H/D exchange, while the purple deuteron at the stereocentre was incorporated using deuterative microbial reduction.
This work was performed in collaboration with Brightspec, Inc., who employed the emerging spectroscopic technique of Molecular Rotational Resonance (MRR) to validate measurements of enantiopurity and deuterium distribution from the work carried out at the NDF.
The benefits of combining synthetic chemistry and microbial transformations for enantioselective synthesis of important chiral molecules was demonstrated in this study, providing methodology that can be further built upon to enable more accessibility for such molecules for research and industrial applications. Synthetic biology techniques may potentially be applied for optimisation of microbial strains for a given substrate for reductive deuteration.
Reference:
Carl Recsei, Marina Cagnes, Robert Russell, Reilly E. Sonstrom and Tamim Darwish (2025)
Studies in enantioselective microbial deuteration, Org. Biomol. Chem. 23:3380-3392
Enquiries
Please direct any enquiries to Tamim Darwish, NDF Leader
Acknowledgments
The National Deuteration Facility is partly supported by the National Collaborative Research Infrastructure Strategy – an initiative of the Australian Government.
Enabled by

