Published on the 7th November 2017 by ANSTO Staff
Japanese researchers have used nuclear techniques at ANSTO to characterise the properties of a new family of materials which they developed for use as solid oxide fuel cells, sensors and oxygen separation membranes.
Devices such as solid oxide fuel cells produce electricity through an electrochemical process from fuels including hydrogen, ethanol and biofuels. The technology helps address energy and environmental challenges because of superior properties and low emissions.
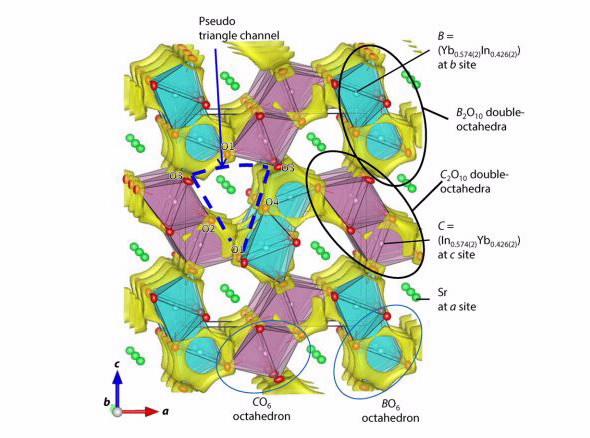
Scientists from the Tokyo Institute of Technology have synthesised and characterised a pure oxygen ion conductor, strontium ytterbium indium oxide (SrYbIn04), which demonstrated high oxide ion conductivity and low electrical conductivity.
The authors, who have published the study in the Journal of Physical Chemistry C, believe that no one has previously investigated this material as a medium for conducting oxygen.
Most oxygen ion conductors are mixed conductors, making them unsuitable for many applications.
The new family has some structural similarities with the CaFe2O4 type materials, but contains no transition metal cation, making it a pure oxygen ion conductor.

The partially disordered crystal structure consists of three cation sites: a strontium cation and two types of double octahredra cations with ytterbium and indium.
“The material had to be an insulator that oxygen could run through easily using minimum energy,” said co-author Echidna instrument scientist Dr James Hester.
Because the ionic radii of Sr2+, Yb3+ and In3+ are larger than those of Ca2+ and Fe3+, there is lower activation energy required for oxygen ion conductivity than CaFe2O4 type materials.
The researchers used a combination of techniques in their investigation, including neutron scattering on the Echidna instrument at ANSTO’s Australian Centre for Neutron Scattering at a range of temperatures from room temperature up to 1273 K.
As an instrument scientist, Hester configured the instrument and furnace, and ensured that the experiment then ran smoothly.
In-situ neutron-diffraction experiments can establish a direct connection between the evolution of crystal structure in a material with property response, such as oxygen permeability.
“We wanted to see how the structure behaved at high temperature to find a path that the oxygen ions might be moving along” said Hester.
The investigation suggested that oxide ions move along the edges of columnar double octahedra in one direction, at one point passing through a bottleneck..
It is just over one atom across but sufficient for oxygen to pass through,” said Hester.
The researchers plan to investigate if oxide ion conductivity can be further improved by doping, a different degree of cation order/disorder and/or adopting larger cations.
“When you start substituting atoms and adjusting, you might be able to go further,” said Hester
“This is a very productive group from Tokyo who come here regularly for neutron experiments. We made the instruments available in the period after Japan closed all its nuclear reactors.”
http://pubs.acs.org/doi/pdf/10.1021/acs.jpcc.7b07911