Researchers in a large international collaboration used beamline instruments at the Australian Synchrotron to determine the three-dimensional structure of key proteins that are regulators of cellular degeneration.
In a paper published in the prestigious journal Science, research led by Prof Bostjan Kobe at the University of Queensland and affiliated groups has clarified the composition and function of the SARM1 protein, which has a role in the death of neurons in humans.
SARM1 acts to breakdown a signalling molecule known as NAD+, a process that leads to degeneration of neurons (nerve cells). The same pathway occurs in plants involving a different protein (RUN1) and a different target.
The investigators used a combination of structural biology, biochemistry, neurobiology, and plant science at Australian, English, and American universities and organisations to elucidate the complex molecular activity that leads to cell death.
There is potential benefit from this research to bring progress on neurodegenerative diseases and plant biosecurity.
“One of our Macromolecular Crystallography (MX) beam lines, MX2, at the Australian Synchrotron was important in this study,” explained MX instrument scientist Dr Daniel Eriksson, a co-author on the paper.
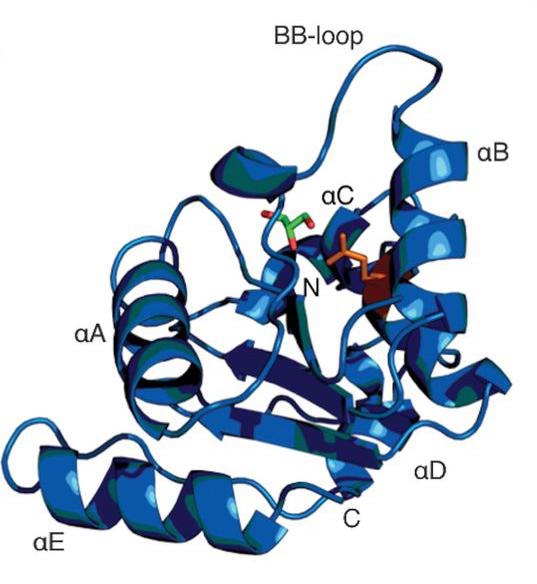
“Although a whole toolbox of techniques is involved in studies like this, macromolecular crystallography is important in deciphering the crystal structure of a complex protein molecule. Once you have the structures, you make better headway in understanding mechanisms at work.
Schematic diagram of hSARM1TIR
“MX2 can rapidly process samples with great precision, allowing you to make an assessment if a sample is worth further investigation. We had to screen a significant number of samples to solve the structures. Eight 3D structures were published in the paper and made available via an open access database for further study.
“By introducing very small changes in the protein, such as a single mutation or introducing a different ligand, you can understand the complex modes of interaction between parts of the protein.
“As some of the changes may be quite subtle, you need high resolution, very high quality data in order to pinpoint those very minute differences."
“In this case, there was evidence that cleavage of specific nucleotides activated a chemical cascades that led to degeneration and cell death,” said Dr Eriksson.
Prior to coming to the Australian Synchrotron as a beamline scientist, Dr Eriksson had a postdoctoral research fellowship in the lab of Prof Bostjan Kobe at University of Queensland, along with and Dr Thomas Ve, now at Griffith University, and Dr Simon Williams, now at the Australian National University.
“I was involved in these experiments because of my close research association with some of the group members, and my expertise in data collection and analysis,” said Dr Eriksson.
Small angle X-ray scattering was also undertaken on another Australian Synchrotron beamline as part of the investigation to elucidate the in-solution behaviour of some of the proteins.
The connection between fundamental cellular processes in both humans and plants is attributable to our common evolutionary origin.
“All life was once one and the same,” said Eriksson.
With plants, important considerations are how the plant behaves when it is attacked by a pathogen and what resistance mechanisms it can bring to bear.
Members of the team included scientists from University of Queensland, CSIRO,Australian National University, Griffith University, University of California Berkeley (US), University of Cambridge (UK), Disarm Therapeutics (US), and ANSTO.
Read more on the University of Queensland website.