A new study published in Nature Communications has shown that, rather than being discarded, plastics can be transformed into valuable carbon nanomaterials that help solve both energy and environmental challenges.
Plastics are one of the world’s most persistent waste problems — durable, difficult to recycle, and increasingly polluting our environment.
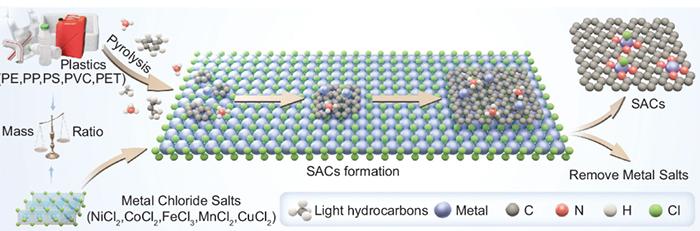
The research team, based at Adelaide University, demonstrated a universal and scalable method to upcycle common plastics — including PET, PVC, polyethylene and polypropylene and their mixtures — into single-atom catalysts (SACs).
These advanced materials contain metal atoms anchored and isolated in a graphene substrate, making them highly efficient in chemical reactions. SACs produced from plastic waste showed excellent performance in breaking down diverse micropollutants in water and in boosting clean-energy technologies such as batteries and fuel cells.
At ANSTO’s Australian Synchrotron in Melbourne, researchers used X-ray Absorption Spectroscopy (XAS) to probe the atomic-scale structure of the catalysts. These measurements confirmed that the metals were not forming nanoparticles but were dispersed as single atoms, chemically bound within the carbon framework in the favourable coordination environment — the secret sauce to their exceptional performance.
“This project highlights how advanced characterisation at the Synchrotron enables breakthroughs in sustainability,” said Dr Bernt Johannessen, Senior Scientist at the Australian Synchrotron and co-author of the study.
“By revealing the atomic structure of these new catalysts, we helped the team understand why they work so well and how to scale the method. The XAS technique is a uniquely powerful tool in studies like these, because it can clearly distinguish between nanoparticles and truly single-atom sites, and we are seeing a surge in demand from researchers worldwide working in this area,” he added.
First-author Dr Shiying Ren from Adelaide University added, "Our work shows that plastics, which are usually considered a waste and an environmental burden, can actually be a valuable resource for making advanced catalysts. This approach opens a sustainable pathway to address both plastic pollution and the demand for new materials.”
A/Prof Xiaoguang Duan, a lead author on the paper, said, “What excites us the versatility of the method, it works across different plastics and mixtures, and produces advanced yet low-cost catalysts that can be applied in water purification, batteries, and beyond. The insights from synchrotron X-rays were essential in proving how the catalysts are structured and why they perform so well.”
The discovery offers a powerful way to give waste plastics a second life as high-performance materials, advancing both a circular economy and next-generation clean technologies. Unlike many recycling approaches, this method works across multiple plastics and even mixtures, producing gram-scale yields that point to real-world feasibility.
The atomic insights made possible by ANSTO’s XAS Beamline were essential to unlocking this sustainable solution. By confirming that the metals were truly present as isolated single atoms, XAS provided the evidence needed to understand why these catalysts are so effective.
“This is a great example of how our capabilities directly support high-impact sustainability research,” Dr Johannessen added. “The Adelaide group, led by Professor Shaobin Wang and A/Prof Xiaoguang Duan, are one of the most productive in the field, and our collaboration shows how synchrotron science can accelerate innovation in environmental and energy technologies.”
This breakthrough is part of a growing body of work by the Adelaide team and collaborators. In a related Nature Communications paper, the researchers reported a complementary salt-templated phase-change strategy that creates a wide library of single-atom and multi-atom catalysts with tailored atomic environments. Together, these studies highlight how new materials can be rationally designed for environmental and energy applications. And how synchrotron characterisation helps make it possible.
DOI: https://doi.org/10.1038/s41467-025-63648-z
Thanks to Dr Bernt Johannessen for his contribution to the content for this article.