This research is the latest in a series of published results that included investigations using macromolecular crystallography beamlines at ANSTO’s Australian Synchrotron.
An international team of researchers has characterised an important interaction that helps the SARS-CoV-2 virus invade human cells.
Scientists from the QIMR Berghofer Medical Research Institute, Charles Sturt University, and the Université Paris-Est Créteil and Monash University have published findings that offer new possibilities for disrupting replication of the virus.
Viruses work by hijacking the cells of their hosts to replicate. They do this by entering cells and releasing their own RNA into the nucleus of the cell, where new proteins are assembled following those RNA instructions.
Imagine a rogue builder sneaking their own blueprints onto the company ‘to-do’ list and getting a house built for free.
In work published in Cell Discovery, researchers looked at the interaction of the SARS-CoV-2 spike protein with a human protein called ACE2.
ACE2 sits on the surface of cells and when the SARS-CoV-2 spike protein binds to it, both ACE2 and the attached virus move into the cell. Once the virus is inside the cell, it can start replicating.

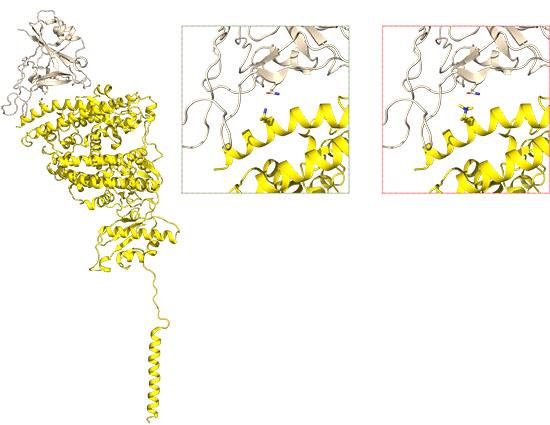
Structure of ACE2 bound to the SARS-CoV-2 spike domain (PDB 6M17) Image courtesy of Prof Jade Forwood, Charles Sturt University
There is, however, a phenomenon, lysine methylation, that determines in part whether the spike protein can bind tightly to ACE2 or not.
Lysine methylation is a bit like the biological equivalent of popping a sticky note on part of a protein. If this sticky note is present on ACE2, strong binding to the spike protein to the human protein is reduced.
If the sticky note is pulled off, the spike protein is free to strongly interact again.
The human enzyme LSD1 is responsible for ‘demethylation’ (pulling off that biological sticky note), which makes LSD1 a therapeutic target for interrupting SARS-CoV-2 replication.
Researchers characterised this interaction through a combination of computational methods, biological assays.
“The structural characterisation of the interaction of the viral protein with the human protein was carried out using our MX beamlines here at ANSTO’s Australian Synchrotron,” said instrument co-lead Dr Alan Riboldi-Tunnicliffe.
“Although only one part of an extensive suite of analytical methodologies, determining the structural interactions of key proteins continues to be an essential tool in research on COVID-19.”
As there are existing drugs that target LSD1 for other purposes, the research opens up avenues to possibly repurpose those drugs to reduce the magnitude of a SARS-CoV-2 infection.