Published on the 17th September 2021 by ANSTO Staff
Key Points
-
Melbourne researchers have made progress in clarifying the molecular interactions that underpin how our adaptive immune cells recognise SARS CoV-2, which causes COVID-19
-
CD8+ T cells, a type of adaptive immune cell, are an important part of the anti-viral immune response in COVID-19 infection and vaccination
-
Investigators used the Microfocus crystallography beamline (MX2) at ANSTO’s Australian Synchrotron to clarify the structure of the interaction between a CD8+ T cell and the Spike protein of the virus and elucidate the role of specific genes
A team of scientists led by Monash University and the University of Melbourne in association with the Peter Doherty Institute for Infection and Immunity have made progress in clarifying the molecular interactions that underpin how our adaptive immune cells recognise SARS CoV-2, which causes COVID-19.
CD8+ T cells, a type of adaptive immune cell, are an important part of the anti-viral immune response in COVID-19 infection and vaccination.
In research published in the Journal ofBiological Chemistry, a team led by Monash Research Fellow Jan Petersen and Prof Jamie Rossjohn of the Rossjohn Laboratory investigated the activity of the Human Leucocyte Antigen (HLA-A2) of T cell receptors (TCR)s and specific gene segments that recognise and respond to the SARS CoV-2 spike protein.
Investigators used the Microfocus crystallography beamline (MX2) at ANSTO’s Australian Synchrotron to clarified in detail how the receptor complex on CD8+ T cells recognises and binds to a specific peptide of the Spike protein and the role of specific immunodominant genes in this response.
Human CD8+ T cells have receptor complexes (TCRs) that recognise epitopes, the part of the SARS CoV-2 antigen (virus) that binds to the receptor complex.
Research by other groups has highlighted that CD8+ T cells respond to about 20 different epitopes across the SARS CoV-2 genome.
Although the adaptive immune response to SARS CoV-2 has been studied widely, Petersen said this may be the first time someone has looked at these CD8+ T-cells from a structural point of view.
“However, because the T-cell response is quite diverse, it is just a little piece of the puzzle,” he said.
“It gives us a way to understand the body’s response to the spike epitope, a protein which plays a major role in the immunisation process," added Petersen.
Following immunisation, which includes some of the Spike protein, the body produces CD8+T cells to fight the virus and emerging variants.
Also, in people who recover from the virus, CD8+ T cells, which may have been depleted, are restored, have a role in eliminating the virus permanently.
Petersen explained that the structural information was a tool to understand what is going on when CD8+ T cells fight the virus.
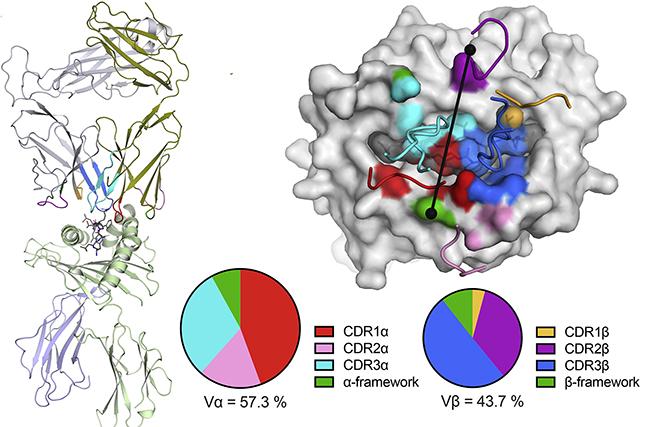
Lead author Priyanka Chaurasia of Monash University undertook measurements using MX2, supported by the MX beamline instrument team, that allowed the team to determine a structural model of the TCR, determined by the HLA-A2 genetic sequence, binding with the Spike protein-peptide of the virus.
The research suggested that this particular T-cell response associated with this epitope was slightly muted and sensitive to changes in the recognized part of the virus.

Structure of the NR1C TCR–HLA-A2S269–277 ternary complex.
“However, there are a number of other epitopes recognized by CD8+ T cells, and they may behave differently,” explained Petersen.
Investigators noted that an understanding of the T cell-mediated protective immune responses to SARS-CoV-2 and potential viral variants is needed to provide insights into how SARS-CoV-2 continues to adapt to human immune responses and our ability to control this evolving pathogen in the long term.
“In evolutionary terms, the virus has just started to evolve in humans, so there are variants, some more infectious and some could further adapt to evade immune responses. So, it is important to stay on top of this and build up an understanding of how the virus might evade T cell responses,” said Petersen.
The MX beamline and other beamlines at the Australian Synchrotron continue to give priority access for COVID-19 related research.
“Australia is very fortunate to have so many productive and energetic teams working on this problem. Our MX beamline is particularly helpful in solving structures of complex molecular interactions, such as immune response.
“We have samples from Monash and other institutions constantly shuttling back and forth to the beamline. This should give everyone confidence that science will continue to help us overcome this pandemic,’ said Dr Rachel Williamson, Principal Scientist.



