The High-Performance Macromolecular Crystallography beamline (MX3) is capable of providing high-flux, microfocus X-rays for small and weakly diffracting protein and chemical crystals.

The High-Performance Macromolecular Crystallography beamline (MX3) is capable of providing high-flux, microfocus X-rays for small and weakly diffracting protein and chemical crystals.
From run 2 2025, MX3 is accepting users for robot mounted single pin and in-tray experiments.
Jan 2026
MX3 had two successful runs in 2025 ramping up user operations. The addition of remote access, in-tray screening and improvements to the user interfaces and reliability of operations were of focuses for 2025. Run 1 of 2026 will be the first run with a full user load, and we look forward to continuing to add capabilities throughout the year.
March 2025
The first expert user experiment was conducted on MX3! Assoc. Prof Ruby Law and Honours student Taru Panjikar from Monash University collected data on peripheral membrane protein crystals. The first experiment of many to come!


February 2024
After last year's installation of the Photon Delivery System, endstation hardware, utilities, and cabin furniture and computers, MX3 is ready to take beam!
While the final stages of the regulatory processes are undertaken, the MX3 team has been focusing on software development. Several important tools are being developed in-house: MXPrism is the tool scientists will use to organise and rank their data, and ScreenShot will allow researchers to send crystallisation trays to MX3 for screening. Additionally, the MX3 software team is developing tools to aid in 'hot commissioning', the process of optimising the X-ray beam's passage through the optics to the sample. Our hope is that these tools can find application in other beamlines too.
The MX3 team will be looking for expert user groups to test the new equipment following hot commissioning. If you are interested in being an expert user, please get in touch with the team at AS-MX3@ansto.gov.au.
October 2023
The second half of 2023 has been very busy for MX3; the hutches and user cabin have been constructed, the Photon Delivery System arrived and was installed, and the hardware being commissioned in the test cage could finally be moved into its permanent home.
The result? MX3 is starting to look like a real beamline! The team has been testing hardware and software, preparing for safety sign-offs, and getting prepared to take first light.

February 2023
At the end of 2022, the MX3 team saw the arrival of the in-vacuum undulator, the MD3 goniometer from Arinax, and the ISARA robot from Irelec. Over the next few months and with the expertise of the equipment vendors, the goni and robot were set up in the MX3 staging area. Having this equipment up and running outside of the hutch gives the MX3 software team valuable time to work on experiment automation.
The user cabin is being constructed, and utilities are starting to go into the hutches, ready for the photon delivery system to be installed later in the year.
November 2022
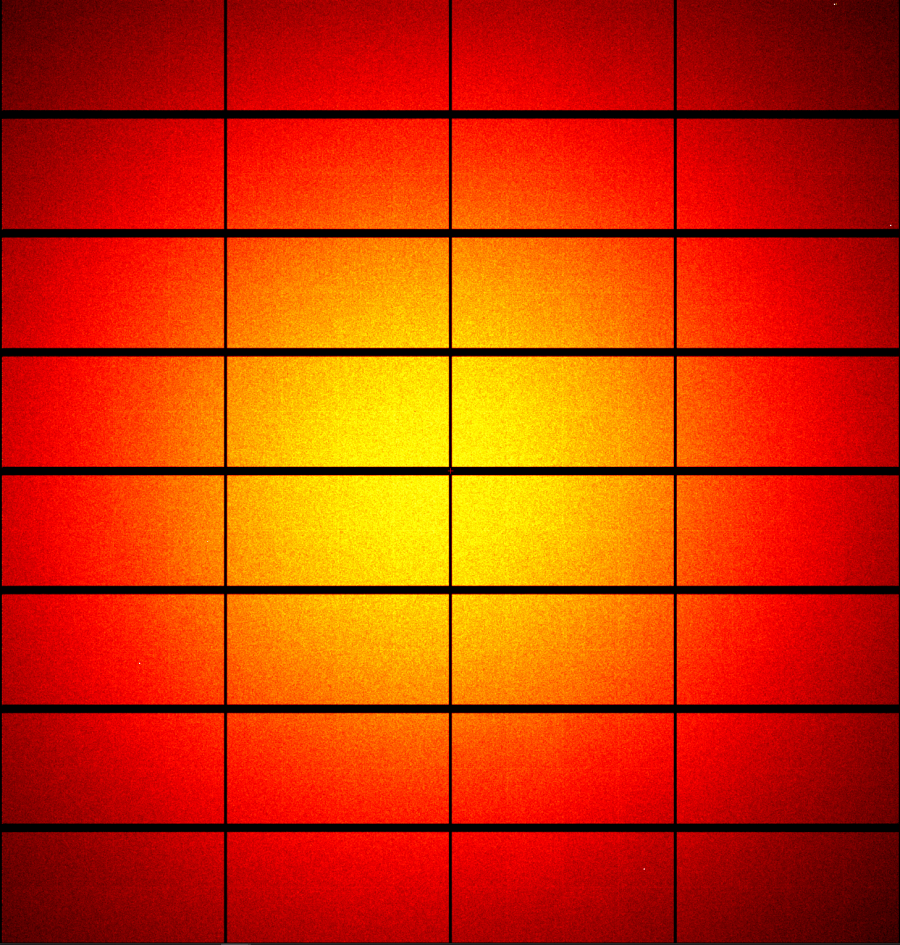
Hardware has started to arrive, and it feels like Christmas has come early! MX3 has just received its Dectris Eiger2 16M detector and several huge crates from Arinax containing the MD3 goniometer and its various widgets.
And here is the first image taken with the new detector. May it collect many more in the name of great science!
More information about MX3 in the video below:
And a timelapse of the MX3 hutches being constructed:
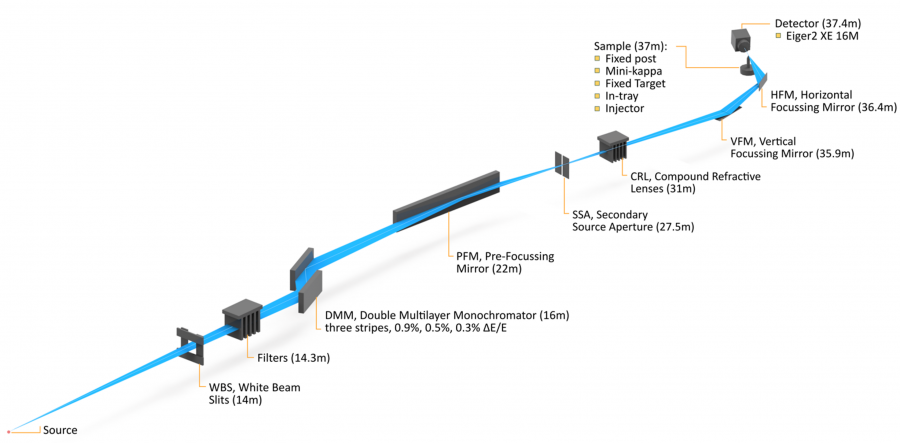
The High-Performance Macromolecular Crystallography beamline (MX3) is capable of providing high-flux, microfocus X-rays for small and weakly diffracting protein and chemical crystals. The beamline provides three modes of operation: goniometer, serial crystallography and in-tray screening. The beamline is powered by a 3m in-vacuum undulator. The optical system will maximise flux at the sample position and the use of a secondary source aperture allows for rapid changes in beam size.
Beam size is adjustable by use of Compound Refractive Lenses, which allow for rapid changes to the beam footprint without moving any reflective optics. In order to produce the required high flux the beamline utilises a double multilayer monochromator. This increases the bandpass (energy width) and brightness of the X-ray beam; however, this will mean that Multi-wavelength Anomalous Dispersion (MAD) experiments are not feasible. The beamline specialises in high flux, high-speed data collection on microcrystals with a high degree of automation for crystal location and data collection.
The MX3 beamline accepts crystal samples in several formats.
Sample shipping for robot-compatible pins is identical to other MX beamlines at the synchrotron and remote access is available. In-tray screening is available.
The core measurement capabilities of MX3 include:
“Standard” MX collection on robot mounted single crystals. Due to the high flux of MX3, an attenuated beam will be required to collect a full dataset from a single crystal, or multiple partial datasets merged from several crystals.
Robot mounted iIn-tray screening using a tray holder on the goniometer. Automation is in development.
High throughput fixed target collection using silicon chip-based sample holder. Chips will be loaded offline, aligned on the beamline (separate fixed-target stages) and collected.
Crystals are introduced to the beam via a sample injector. Several designs are being considered but the production system will deliver crystals to the beam with high speed and low background scattering to allow data collection and structure solution from multiple micro-crystals.
MX3 allows for experiments that are currently only marginally feasible or impossible on the existing MX1 or MX2 beamlines (such as small GPCR crystals or the use of fast injectors/serial crystallography). This beamline will enable Australian researchers to keep pace with the advances in the field of synchrotron-based crystallography. MX3 addresses the following current and predicted needs of the crystallographic community in Australia, New Zealand and Asia:
Operation of MX3 will allow MX1 to specialize in low-energy biological macromolecular crystallography (MX) and chemical crystallography (CX) collection. The current MX1 and MX2 beamlines cater for the needs of CX users providing relatively high flux small beams (few CX samples are flux limited). Future new capabilities for synchrotron-based chemical crystallography will be focused on in-situ environments such as furnaces and pressure cells. CX users will also have access single crystal diffraction capabilities using the Advanced Diffraction and Scattering (ADS) beamline where higher X-ray energies will allow for effective use of large experimental systems such as furnaces and pressure cells.
MX3 will provide the ability to collect single crystal diffraction data at very high speed; this is needed in several instances:
The MX3 beamline will be particularly useful in the study of proteins that are difficult to purify or have complex or changeable structures; for example, proteins that are located in or associated with membranes. Normally, such proteins present enormous challenges with respect to crystallisation and therefore structure determination. This is particularly true for receptors, which are present within membranes. Protein receptors are a crucial part of all cell signalling pathways, where dysfunction can lead to a range of diseases including many forms of cancer. Most membrane receptors exist as complexes of smaller proteins, called subunits, which can only be purified in very small quantities as microcrystals. These crystals cannot be studied on the current MX beamlines available at the AS but could be analysed using MX3.
A specific disease case study relates to Alzheimer’s disease. This neurodegenerative disorder is characterised by the presence of extracellular amyloid plaques in the brain. In the quest for a cure for this condition, Australian scientists are studying precursor proteins that are responsible for the peptides that form the plaques and have been shown to be toxic to neurons. Only very small crystals of these targets have been produced, which are too small to study with the current AS beamlines.
Another example is the study of biological rotary motors. These are fascinating protein assemblies that are found in the body and which are inherently difficult to study due to their trans-membrane nature and size. Once again, facilitation of this study is critical as these structures are often implicated as the cause of diseases, such as heart disease. A high-brilliance microfocus beamline, such as MX3, will allow scientists to expose only small parts of a crystal that might be better ordered than others. Currently Australian researchers from the Victor Chang Cardiac Research Institute have been forced to work at the Advanced Photon Source in the USA, and the European Synchrotron Radiation Facility in France in order to undertake such studies.
Viruses are responsible for a wide range of diseases in humans and other animals, ranging from the COVID-19, common cold to ebola, AIDS and even some types of cancer. Aspects of viral structure and behaviour have been studied using crystallography, but this normally involves small, weakly diffracting crystals which will be able to be studied using MX3. For example, insect viruses can remain active for years in the environment using a type of armour formed of crystals called viral occlusion bodies. Understanding these bodies would have important implications for the development of new bio-insecticides and vaccines. A wide range of other applications exists in the life sciences, and this need is increasing as scientists turn to the more challenging but potentially more significant health problems that this beamline will make possible.
A wide range of materials only form incredibly small crystals that cannot be studied using existing beamlines. Examples include microporous and mesoporous materials, hydrogen storage materials, novel metal oxides and ceramics, superconductors, minerals, 'smart' materials, piezoelectric materials, novel magnetic materials, photonic devices, information storage materials, molecular switches and sensors, biomimetic materials and pharmaceutical materials.
The MX3 beamline is designed to provide the highest flux in a microfocus MX beamline available at the Australian Synchrotron. This is achieved through the use of an in-vacuum undulator (IVU) along with the use of a double multilayer monochromator (DMM) and horizontal secondary source aperture (SSA) provide the high flux and small focal size, respectively.
Beam stability is a key design requirement with the only vertical optical element being the vertical focusing mirror (VFM) in concert with the horizontal focusing mirror (HFM) adjacent to the sample. This should reduce the impact of vibration on beam position given the use of a horizontal DMM and horizontal SSA.
Thermal stability in the endstation is critical with a design goal of 0.1°C rms in the endstation and optics. Humidity will be controlled and as low as 25% for routine robot operations.
Equipment includes:
| Energy range | 10 to 19 keV | |
| Number of endstations | 1 | |
| Source | Type | 3 m In-vacuum undulator (IVU) |
| Pole pairs | 172 | |
| Period | 17.2 mm | |
| Total power | 2.52 kW | |
| Optics | ||
| Horizontal double multilayer monochromator | Ru/B4C with 3 sets, 0.9% bandpass, 0.5% and potentially 0.3%. | |
| Horizontal prefocussing mirror (PFM) | 600mm long at 3mrad | |
| Horizontal Secondary source | 170 micron gap (FWHM) for full beam. Down to 34 micron gap for 2x2 micron beam. | |
| Vertical focussing mirror | 400mm long at 3mrad | |
| Horizontal focussing mirror | 400mm long at 3mrad | |
| Spot size at the sample position | Full beam 8-2 microns (FWHM) at > 6e13 ph/s | |
| Minimum beam 2x2 microns (FWHM) at > 1e13 ph/s |
| Scheduled Completion Dates | Key Project Deliverables |
| July-2019 | Project Started |
| 2020 | Design Completion |
| 2022 | Procurement Completion |
| 2022 | Equipment Installation Completion |
| 2022-2024 | Cold Commissioning Completion to 1st Light |
| Run 1 2025 | Hot Commissioning Completion, includes expert users |
| Run 2 2025 | First User Experiments; Beamline fully commissioned over the next 12 months |