
Published on the 20th October 2021 by ANSTO Staff
Key Points
- ANSTO is a specialist centre for batteries research offering both capabilities and expertise
- Nuclear and accelerator techniques can be used to characterise the properties of materials for batteries
- Experiments are undertaken using operating batteries for the most accurate understanding of cycling processes
Prof Vanessa Peterson from the Australian Centre for Neutron Scattering and Dr Chris Griffith of Minerals participated in the launch of ANSTO's Innovation Series in association with Science meets Business in a session on The Future of Battery Power.
The following article highlights some of the work undertaken at ANSTO in support of batteries research.
The 2019 announcement of the Nobel Prize in Chemistry to three scientists whose combined efforts produced the first lightweight, rechargeable battery was not a surprise to materials researchers around the world.
“These Nobel recipients are materials researchers. Materials characterisation methods bring an understanding at the molecular and atomic level, that make advances such as the lithium-ion battery possible,” said Prof Vanessa Peterson, Senior Principal Research & Neutron Instrument Scientist, Powder Diffraction and Leader, Energy Materials Research Project.
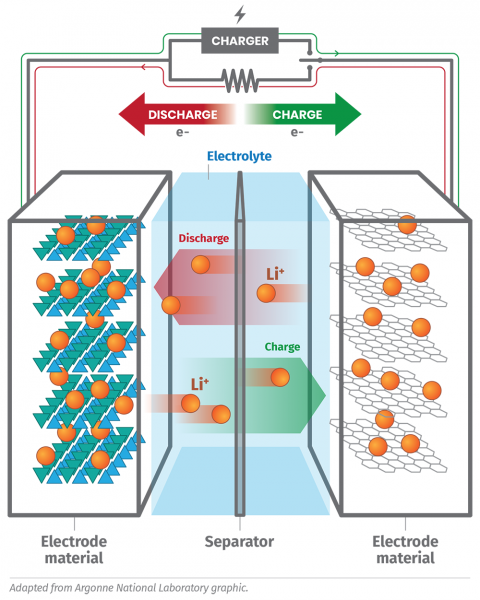
Stanley Whittingham discovered that you could push lithium ions into a material reversibly as the basis of a battery. John Goodenough developed the concept of using a cobalt oxide electrode to reversibly host lithium ions, creating a higher voltage battery, and Akira Yoshino showed that graphite could also be used as an electrode alongside the cobalt oxide. Together, graphite and cobalt oxide are the materials that were commercialized in the first lithium-ion battery. They are still used today.
“You can’t see this process with your eyes, so we need advanced methods to understand the origin of the chemical properties in functional materials, and this is key to improving them.”
Peterson has been working on lithium-ion and other types of materials used in energy storage and delivery systems since she started an energy materials research program in 2009.

It was under this program that the very first measurement of lithium ions being incorporated and released from electrode materials within a whole working modern lithium-ion battery in real-time was made, taking advantage of the high-intensity neutron diffractometer Wombat, which Peterson co-operates as reported in the Journal of Power Sources. This battery contained the same cobalt oxide and graphite materials for which the 2019 Nobel prize was awarded.
"The measured data were given to Neeraj Sharma, then a postdoc working in Peterson’s energy materials project, and the mechanism for the reversible lithiation of these electrodes was determined within the whole functioning battery – therefore accurately representing the real working mechanism. "
“That was a substantive advance at the time,” said Peterson.
“It is important to determine how the atomic structure responds to an electrochemical process, particularly changes in composition that occur. “
In a recent paper in Advanced Materials, Peterson, alongside her postdoc and collaborators from the University of Wollongong, outlined the method - how neutron powder diffraction can be used to study reactions and associated structural changes of electrode materials within whole functioning batteries.
“The neutron diffraction method can be tailored to get more detailed information.”
“In batteries, you need a material that can accommodate a large number of charge-balancing ions, which relates to the battery capacity; can quickly release and then reversibly store these ions, which relates to the battery rate capability; and can sustain this process many times reversibly, which relates to the battery lifecycle.”
“If a material has substantial changes in structure or volume when it takes in or release ions; this can lead to stress and strain and degradation of the material, reducing the battery lifetime and capacity.”
“Computational calculations can help in predicting what structures are stable mechanistically for different material compositions.”
“Neutrons are essential for studying battery materials. Most modern battery electrodes use more than one transition metal element, such as cobalt, which change their valence in accordance with the battery state of charge.”
“These elements have similar numbers of electrons, which make them indistinguishable to X-rays.”
“However, neutrons can provide good elemental contrast and are usually necessary to determine the arrangement of these different elements within the material structure.”
“Not only was the first real-time measurement of the changing electrode atomic structure within a whole battery made using neutrons at the Australian Centre for Neutron Scattering (ACNS), but the very first determination of the changing location and concentration of lithium ions within electrodes from within a whole functioning battery was also made as part of the energy materials research project at the ACNS."
“The methods developed here allow both the identification of phases and compositional changes in the battery electrodes, as well as very detailed information about exactly where the lithium ions are in those electrode materials and how those materials respond to that change.”
“An example of that was research on a lithium titanium oxide electrode material, which is structurally more stable than graphite, and we were the first to explain in detail why it has that property. This was published in the Journal of Power Sources
In addition to the research on lithium-ion batteries; the team also investigates other types of batteries that can reversibly host ions, such as sodium and potassium ion batteries.
Dr Christophe Didier, a post-doc working with Peterson at the ACNS and shared with Peterson’s University of Wollongong collaborators, published work in Advanced Energy Materials providing structural insights into layered manganese oxide electrodes for potassium-Ion batteries.
“In this case, we were able to use X-rays on an operating battery at the Australian Synchrotron, because potassium has a lot more electrons than lithium.”
These results again confirm the importance of understanding the detailed structural evolution that underpins performance that will inform the strategic design of electrode materials for high-performance potassium ion batteries.
“We do have many collaborators but we are always interested in new projects. Because we are knowledgeable in the materials themselves, we can contribute to the selection of suitable materials as well as leading the characterisation effort.
“Often we learn something about the structural transformation that might help improve the properties of the material or identify other classes of materials that may also have properties useful for that application.
“Creating a global energy system that is both environmentally and economically sustainable is unquestionably one of the largest challenges facing the scientific and engineering communities. We are here to contribute to that effort, said Peterson
Using synchrotron light to study batteries
Powder diffraction instrument scientist, Dr Qinfen Gu (pictured below) at the Australian Synchrotron has assisted many investigators working on lithium and other types of batteries.

More than 50 per cent of proposals accepted for the powder diffraction beamline are related to batteries research.
“Although lithium is a very light element that only scatters X-rays weakly, it is still possible to use x-ray powder diffraction on these batteries while they are operating.
“The synchrotron light source is extremely powerful and can deliver a high resolution.”
It provides valuable information about the complicated electrochemical reaction mechanisms in lithium-ion batteries when combined with other techniques.
This includes monitoring the changes of crystal structure, electronic structure, chemical composition, and morphology of electrode materials in electrochemical cells during operation.
Collaborators from the University of Wollongong, Adelaide University and the University of Queensland are frequent users of the X-ray powder diffraction beamline for battery research.
“Many are working on the development of new materials for batteries as well as developing new types of mechanisms, such as sodium-ion batteries.” said Gu.
There is also a range of other instruments and techniques at the Australian Synchrotron that can be used in battery research, including soft X-ray absorption spectroscopy for surface/interface studies and hard x-ray absorption spectroscopy for bulk structure information.
“We fully expect battery research to remain a hot topic for the next 10 years,” said Gu.