
Published on the 19th August 2021 by ANSTO Staff
Key Points
-
Method used by Dutch food scientists can be used in the design of new oleogel systems
-
Oleogels are used as healthy substitutes for saturated and trans fats
-
Neutron scattering at ANSTO was used to characterise the components of the oleogel
There is a lot of interest in the food industry in developing an alternative to the use of trans fats, which raise ‘bad’ cholesterol levels and lower ‘good’ cholesterol levels, as well as saturated fats, which elevate ‘bad’ cholesterol levels.
As polyunsaturated fats from plants are liquids, and not always suitable for some foods, one approach is to use a process known as oleogelation. This involves adding something to a liquid fat, such as olive oil, to convert it into a semi-solid gel.
International researchers have used nuclear techniques at ANSTO - a centre for food materials science - to develop a methodology that could assist in the design of oleogel systems for food applications.
The approach provided insights into the mechanism that enabled molecules to form an oleogel network structure in edible oils.

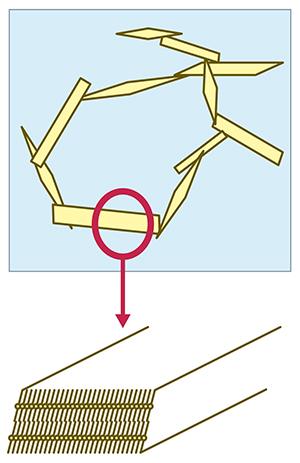
Soft-solid materials (or gels) that can be formed from an edible liquid oil without resorting to the addition of solid saturated fats, can be broadly described as oleogels. The ‘gelator’ within it forms a network structure that immobilises the liquid oil. The properties of oleogels depend on how molecules arrange themselves to provide a three-dimensional structural network. The research team investigated the use of oleic acid in combination with its sodium salt.
(Figure left) Assembly of crystalline gelators in oleogels
In a paper published in Innovative Food Science and Emerging Technologies, investigators from Wageningen University in the Netherlands reported that sodium oleate plays a critical role as the principal oleogelator and produces stable, solid-like behaviour in the oil.
The study was undertaken by A/Prof Elke Scholten, Chair of Physics and Physical Chemistry of Foods group at Wageningen University, and Dutch food scientist Steven Cornet.
However, the presence of oleic acid also contributed to the overall gel properties. Varying the ratio between oleic acid and sodium oleate could provide control over the melting behaviour of the oleogel, as well as its mechanical properties.
Investigators used a combination of techniques, including rheology, microscopy as well as small-angle neutron and X-ray scattering and ultra-small angle neutron scattering at ANSTO.
Co-author Prof Elliot Gilbert, Leader of ANSTO’s Food materials science project said, “Small-angle neutron scattering is able to discern the different contributions that each component makes to structures on both the nano- and microscale. It is these structures that determine the properties of the gel on the macroscale.”
In the structural study, investigators characterised the components of different edible oils (medium-chain triglycerides, olive oil and sunflower oil) as well as additional concentrations and ratios of oleic acid to sodium oleate.
The investigators noted that, from a food perspective, although the high amounts of oleic acid or sodium oleate studied would be unsuitable for direct use in a food product, the knowledge gained from neutron scattering in understanding how the oleogel properties can be fine-tuned will be invaluable in the creation of future food-relevant oleogels.
Listen to a recent Singularity U podcast that features Prof Gilbert talking about the structure of food.
DOI: https://doi.org/10.1016/j.ifset.2021.102763
Listen to the full version of a recent Singularity U podcast that features Prof Gilbert talking about the structure of food. Extract above on the subject of fats from podcast.