
Stable production of hydrogen from seawater
Efficient electro-catalysis of hydrogen from seawater represents a low-cost, abundant source of clean energy. However, a lack of seawater-stable catalysts have hindered its development. In this study, nickel surface nitride nanoparticles were shown to be an effective and robust catalyst. Using the Soft X-ray Beamline at the Australian Synchrotron, the surface chemistry responsible for the catalytic activity was revealed, paving the way for industrial scale hydrogen production from seawater.
Our research

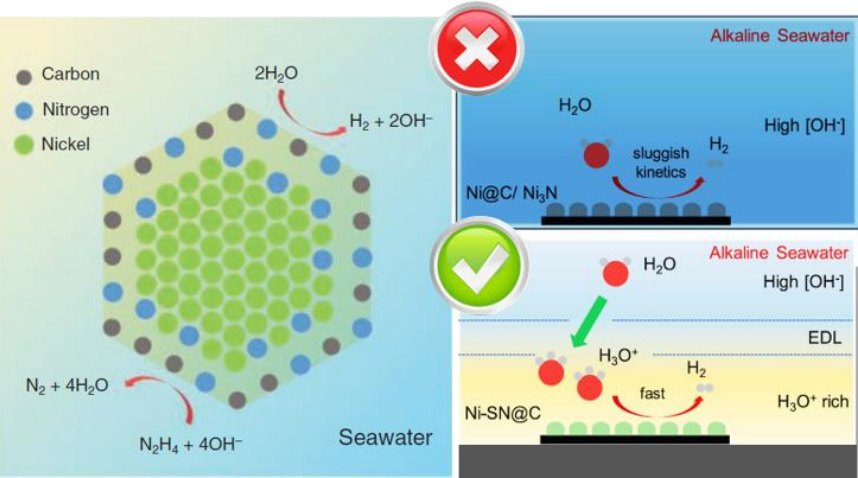
Nickel surface nitride nanoparticles are a more effective catalyst for hydrogen production from seawater, compared to metallic nickel
The efficient industrial scale production of hydrogen from seawater using an electrocatalytically driven hydrogen evolution reaction (HER) would represent a game changing contributor toward the global supply of abundant green energy. Unfortunately, most conventional noble metal based catalysts for hydrogen production in pure water produce corrosive hypochlorite by-products when used in seawater, which quickly inhibit the catalytic activity and degrade the electrodes.
Recently, researchers from the School of Chemical Engineering and Advanced Materials at the University of Adelaide synthesised a new transition metal nanoparticle compound, Nickel surface nitride (Ni-SN@C), which demonstrated catalytic efficiency for hydrogen generation in seawater comparable or greater to that of typical platinum-carbon (Pt-C) based compounds used in pure water.
In order to optimise the catalytic material efficiency through materials engineering techniques, a detailed understanding of the surface chemistry of the catalyst is required. The researchers used the Soft X-ray beamline to investigate the surface chemistry of the Ni-SN@C nanoparticles at the atomic level. They were able to show that the nanoparticles consisted of a pure metallic Nickel core, with a surface layer of nickel nitride. Importantly, this nickel-nitride layer exhibited reactive dangling bonds which are thought to be responsible for the increased catalytic activity compared to bulk Nickel Nitride (Ni3N).
Our impact
Development of transition metal-based catalysts for the production of hydrogen from seawater is attractive both in terms of cost/sustainability (abundance of transition metals) as well as the stability of the catalyst in the alkaline seawater environment.
These results provide fundamental insights into the surface chemistry engineering required in transition metal based catalysts to achieve large scale, efficient, hydrogen generation from an abundant resource.